[[{“value”:”
Malaria
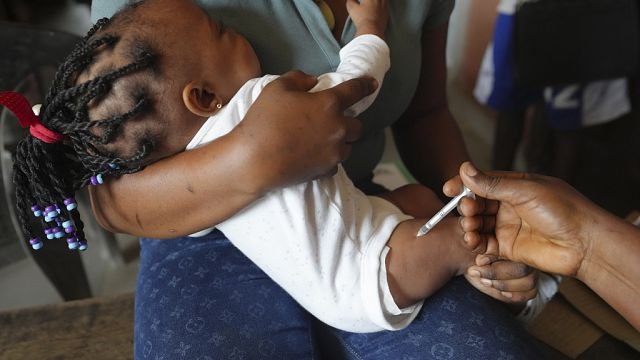
For the first time ever, a malaria treatment has been approved for infants, according to a press release by pharmaceutical company Novartis on Tuesday.
Eight African countries participated in the assessment of the breakthrough medicine and are expected to roll out treatments in the fall.
Until now, there has been no approved malaria treatment for infants weighing less than 4.5 kilograms, leaving a deadly treatment gap, the press release states.
They have instead been treated with formulations intended for use in older children, which may increase the risk of overdose and toxicity. The new treatment has an adjusted dose strength designed specifically for the youngest children.
“Together with our partners, we are proud to have gone further to develop the first clinically proven malaria treatment for newborns and young babies, ensuring even the smallest and most vulnerable can finally receive the care they deserve,” said Vas Narasimhan, CEO of Novartis, the company behind the new drug.
The most recent figures from WHO show that there were 597,000 deadly cases of malaria in 2023 across 83 different countries.
Africa carries a disproportionately high share of the global malaria burden. The region is home to 94% of global malaria cases (246 million) and 95% (569,000) of all malaria deaths. The majority of fatalities are children under 5 years old.
Novartis plans to introduce the treatment on a largely not-for-profit basis to increase access in areas where malaria is endemic starting with the eight African trial countries: Burkina Faso, Ivory Coast, Kenya, Malawi, Mozambique, Nigeria, Tanzania, and Uganda.
Related articles
Most read
“}]] Africanews RSS